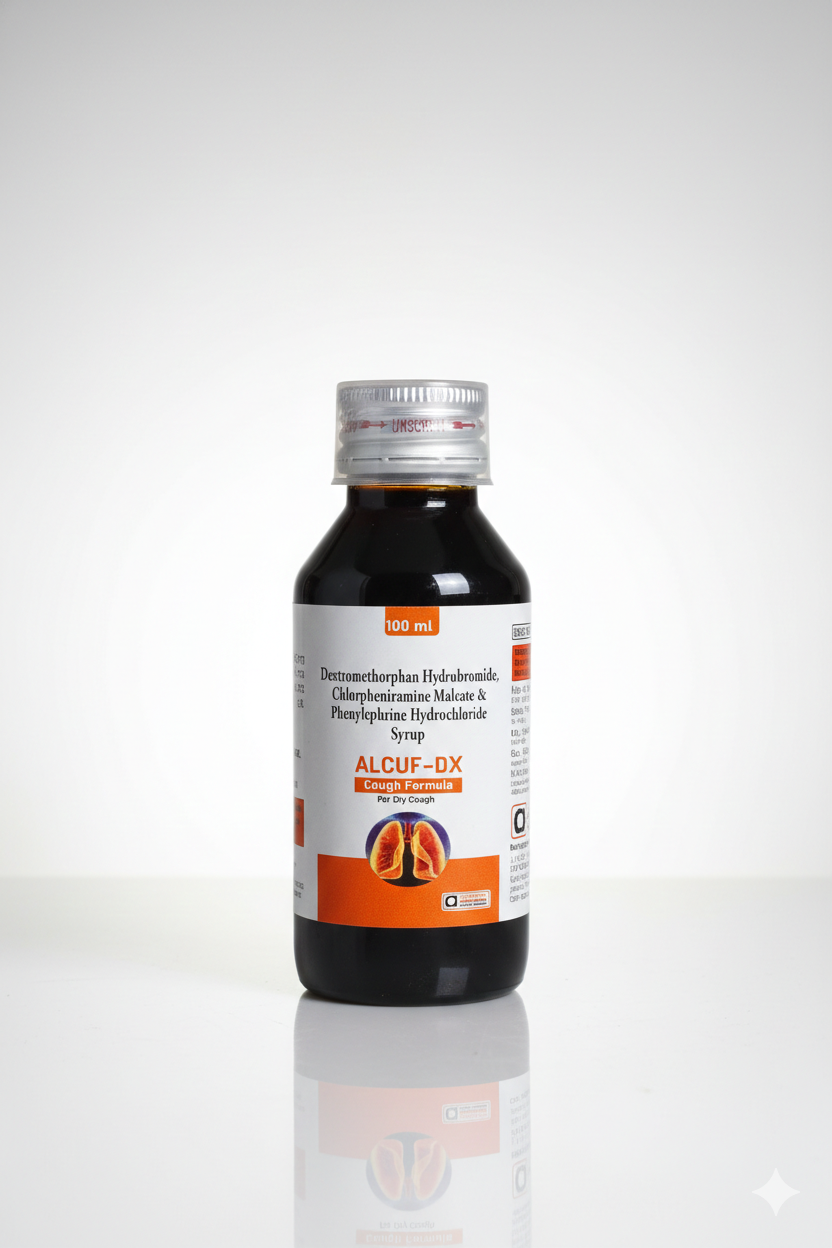
The image shows a 100ml bottle of ALCUF-DX, a combination cough syrup specifically formulated for the relief of dry cough and associated cold symptoms.
Based on the label, here is a breakdown of its components and intended use:
This medication is a triple-combination formula containing:
Dextromethorphan Hydrobromide: A cough suppressant (antitussive) that works by blocking the cough reflex in the brain.
Chlorpheniramine Maleate: An antihistamine that helps relieve symptoms like watery eyes, itchy throat, and sneezing.
Phenylephrine Hydrochloride: A decongestant that helps shrink swollen nasal passages to make breathing easier.
Target Symptom: Specifically labeled “For Dry Cough” (non-productive cough without mucus).
Appearance: A dark-colored liquid housed in a brown (amber) bottle to protect the light-sensitive ingredients.
Cap: Features a silver-colored screw cap with “Unscrew” instructions printed on the seal.
Because this medication contains an antihistamine and a suppressant, you should keep the following in mind:
Drowsiness: Chlorpheniramine can cause significant sleepiness. Avoid driving or operating heavy machinery until you know how it affects you.
Alcohol: Do not consume alcohol while taking this medication, as it can dangerously increase sedation.
Pre-existing Conditions: Consult a doctor if you have high blood pressure, glaucoma, or prostate issues, as Phenylephrine can affect these conditions.
Dosage: Always follow the dosage instructions provided by your healthcare professional or as printed on the back of the label.
Note: This syrup is intended for dry coughs only. If you are coughing up thick mucus (a productive cough), a different type of medicine called an expectorant is usually recommended instead.
There are no reviews yet.